Arrangement of Only the Atoms in a Molecule
Name the different elements found in this compound. 5 What are atoms and molecules together called.
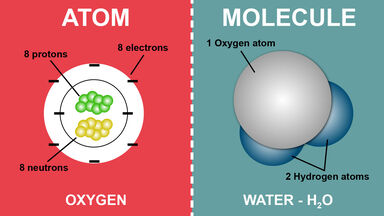
Basic Difference Between An Atom And A Molecule
7 What is the regular arrangement of atoms within a crystalline solid.

. This valence theory is based on. The identification of atoms and the structure of the compound is given by structural formula. If you switch to a different.
9 What is formed when 2 hydrogen atoms are combined with 1 oxygen atom. The shape and geometry of a molecule can be determined using the Valence Shell Electron Pair Repulsion. The arrangement of only the atoms in a molecule or ion.
10 How do atoms make new molecules. In a methane molecule CH4 there are 4 single covalent bonds. 10 When two hydrogen atoms share electrons What is the result.
One atom becomes more electronegative than another atom. 11 Why are two hydrogen atoms bonded together considered a compound. But the formula does not tell about the structure of atom that is how atoms are arranged with each other.
Position isomerism It is due to the difference in position of functional groups. Thus the NH 3 molecule has a trigonal pyramidal shape. - Any nonbonding pairs in the molecule are not part of its description.
Accounts of Chemical Research 1993 26 5. A n _______________ hydrocarbon is a hydrocarbon in which the atoms of each molecule use only single covalent bonds and each carbon atom is bonded to four other atoms. Abat is the 3-dimensional arrangement of the atoms in a molecule.
The diagram shows the arrangement of the outer electrons only in a molecule of ethanoic acid. 2 The same no of paired electrons. Resonance structure of the molecules should not have the following.
No indication is given about how a molecule is existed in space. Is molecular formula of glucose. 3 marks 107 1 Lone pair-bond pair repulsion is stronger than bond pair-bond pair repulsion.
8 Are atoms composed of molecules. It displays the three-dimensional position of the. Their ratio is 12.
In one of them the carbon atoms lie in a straight chain whereas in the other the chain is branched. 4 What shows the arrangement of the atoms in a molecule of a substance. A structural formula provides the most information because it shows not only which elements and how many atoms of them are present in the molecule but it also shows how the atoms are arranged in.
Two atoms attain equal electronegativities. Empirical formula only give us the ratio in which different elements are present in that. - The energy of a resonance structure is always less than any of its canonical form.
C State the H N H bond angle in the NH 3 molecule. E 8 -- 2p where c is the total number of available electrons in the shells of all the atoms in a molecule. Arrangement of these clusters is supposed to constitute the structure of the crystal.
A molecular formula is the representation of the actual number of atoms and not the shape and geometry of a moleculeFor example the molecular formula of methane CH 4 cannot convey the fact that a methane molecule has a tertrahedral geometry. The arrangement of atoms in a substance is the way the atoms are bonded together. 8 What is produced when two atoms combined.
Covalent bonds are the connections that hold atoms together in molecules and we can describe a molecule as simply 2 or more atoms connected by covalent bonds. You can get information about a substances composition from both the chemical formula and the model. 9 How are atoms combined with other atoms to form molecules.
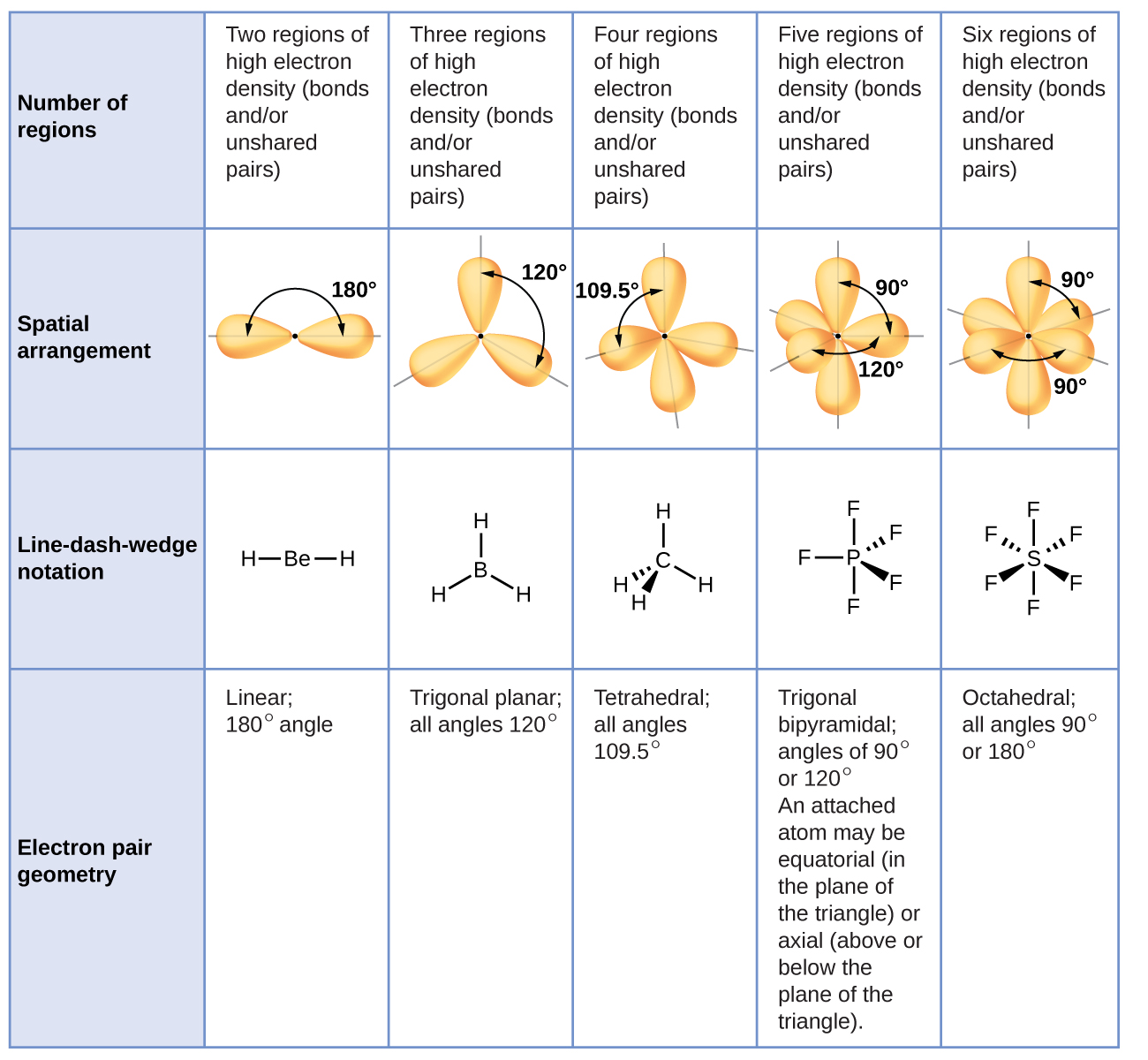
THE ARRANGEMENT OF ELECTRONS IN ATOMS AND MOLECULES. Arrangement of only the atoms in a molecule Electron geometry Tetrahedral for N F 3 Molecular geometry Arrangement of bonding and non-bonding electron domains Electron geometry Trigonal pyramidal for N F 3 Molecular geometry. Very important for reactivity and properties.
The following simple equation. This shows that an iron Fe atom and 2 chlorine atoms are involved with each other. 1 The shape of a molecule is determined only by the arrangement of atoms.
Applying the VESPR Model to Determine Molecular Shapes. Difference in arrangement of C atoms in the chain. The composition of a substance is the combination of chemical elements that make up the substance.
G Identify each term as a description of an electron geometry or a molecular geometry. Account for the value. How many single covalent bonds does each carbon atom have.
Two atoms can be connected by 1 covalent bond single bond 2 covalent bonds double bond or even 3 covalent. Arrangement of bonding and non-bonding electron domains. Thus we can conclude that the formula of a compound indicates all of the following except the arrangement of the atoms in the molecule.
Molecular formula of a compound indicate the different elements and the exact number of different elements present in that compound. One atom pulls an electron from another atom. What is the total number of atoms present in this molecule.
In an octane molecule C8H18 there are 25 single covalent bonds. 1 Identify each term as a description of an electron geometry or a molecular geometry. Further it is usually believed that the arrangement of the crystal molecules is determined by their inherent symmetry and the symmetry of each crystal molecule is unhesitatingly ascribed to the arrange-ment of the atoms within it.
Structure around a given molecule is determined principally by minimizing electron-pair repulsion ie minimizing energy 6 other. 12 What happens when two atoms get near each other that causes them to bond. Ashape can be approximated by valence electrons with the Valence Shell Electron Pair Repulsion Model VSEPR aBasis of VSEPR model.
Atoms molecules and metallic clusters. - The electron pairing should be different in case of the canonical forms. _____ 1 Electron Geometry 2 Molecular Geometry Arrangement of only the atoms in a molecule.
Arrangement of only the atoms. For example there are two isomers of butane C 4 H 10. 6 How is atom arranged.
Between which two atoms is there a double covalent bond. If you switch to a different device you may be asked to login again with only your ACS ID. 1 Energy greater than any of its canonical structure.
A saturated hydrocarbon contains the maximum number of ___________ molecules that a molecule with that number of carbon atoms can hold. Is the nmnber of octets forming the outside shells and p is the number of pairs of. The relative arrangement of atoms in a molecule can be studied by knowing structural formula of the compound.

Molecule Definition Examples Structures Facts Britannica
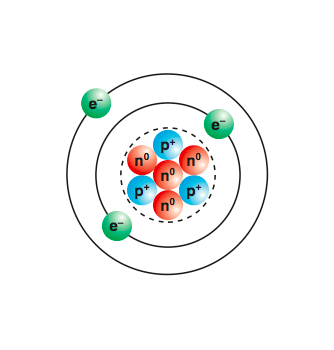
Atoms Molecules And Compounds Manoa Hawaii Edu Exploringourfluidearth
Comments
Post a Comment