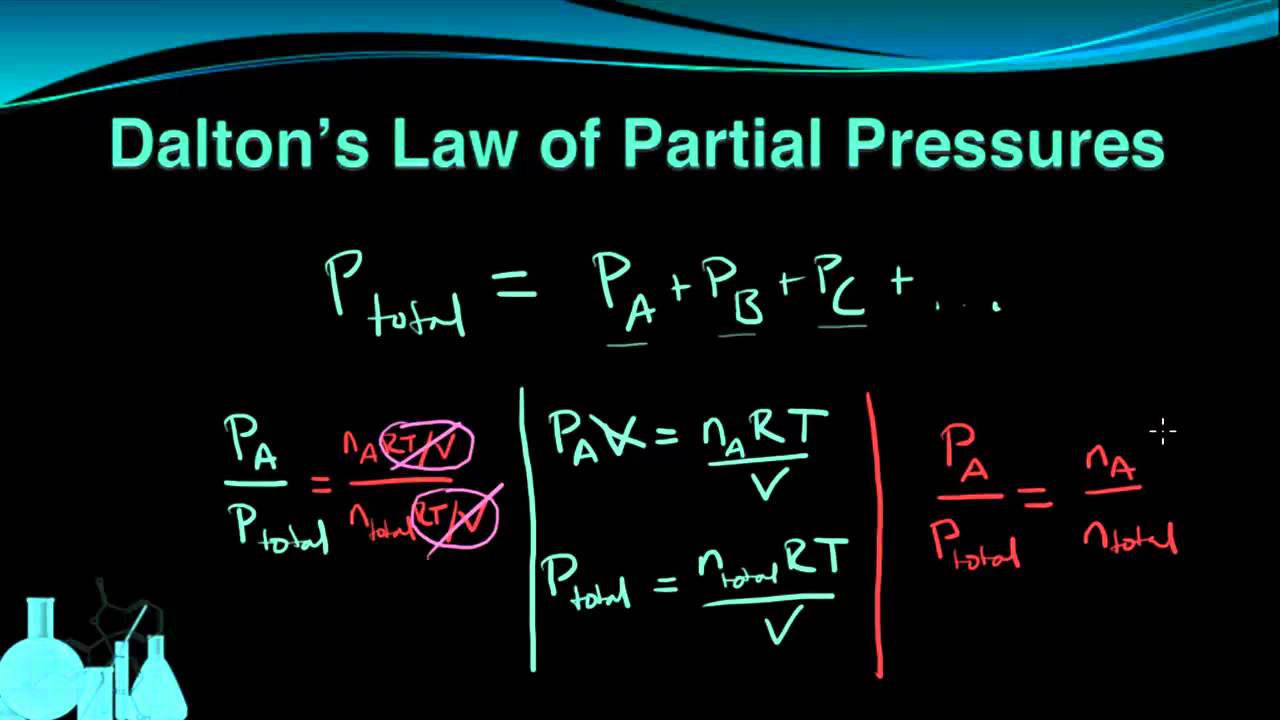
Dalton's Law of Partial Pressure
Daltons law is not exact In this system pressure is not intensive. Daltons Law of partial pressure for moist air can be expressed as.
Chemistry 7 6 Dalton S Law Of Partial Pressures Dalton S Law Chemistry Dalton
It provides the equations plus plenty of examples and practice probl.

. Daltons law of partial pressure was given by English Chemist Physicist and meteorologist John Dalton in 1802. Formulated by John Dalton in the year 1801 Daltons Law or Daltons Law of Partial Pressure states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of each individual gases present in the mixture. Formula of Daltons Partial Pressure.
R 8314 J K -1 mol -1 ideal gas constant. P 1 p 2 p 3p m Partial pressures of the individual gases in the mixture. What is Daltons Law of Partial Pressure.
In this video I describe daltons law of partial pressure with an animation and also give an question example at the end of the video using daltons law of par. The partial pressures of hydrogen oxygen and argon are 020bar 032barand 001bar. From Daltons law of partial pressure Example 2.
Daltons law of partial pressures can be used to calculate total pressure of gases in a container or to find the pressure of a single gas. Daltons Law of Partial Pressure. According to Daltons law of partial pressures the total pressure exerted by the mixture of gases is the sum of the partial pressure of every existing individual gas and every gas is assumed to be an Ideal gas.
In a mixture of gases each individual component gas contributes to the total pressure of the mixture. Calculate the total pressure of the mixture. Daltons law of partial pressures assumes that the gases in the mixture are non-interacting with each other and each gas independently applies its own pressure the sum of which is the total pressure.
What is Daltons Law of partial pressure and Amagat law. Use Daltons law of partial pressures Given a completely evacuated 100-L box at 25 oC enough hydrogen is pumped into the box to make its partial pressure equal to 0463 atm. Partial pressure refers to the pressure that is exerted by one of the gases inside of the container.
This chemistry video tutorial explains the concept of daltons law of partial pressure. P total pressure of air Pa Nm2 pa partial pressure dry air Pa Nm2 pw partial pressure water vapor Pa Nm2 With the partial pressure. Total pressure is a linear sum of partial pressures insofar the ideal gas law is a valid approximation.
Daltons Law of Partial Pressures or Daltons Law states that the total pressure of a gas in a container is the sum of the partial pressures of the individual gases in the container. What is the total pressure in the box assuming ideal behavior. The Daltons law calculator is derived from the law of partial pressures.
Here is a worked example problem showing how to use Daltons Law to calculate the pressure of a gas. In this tutorial you will learn what partial pressure is how to find the partial pressure of a gas and how Daltons Law relates it to mole fraction. The partial pressure of the gas is represented by the symbol P with the symbol of the gas in the subscript.
P1 P2 P3 are the partial pressures of the various gases in the mixture. Daltons Law Core Concepts. Where P 1 P 2 P 3 are the partial pressures of gas 1 gas 2.
Three gases hydrogen oxygen and methane are mixed in a container. The pressure exerted by each gas in a mixture is called its partial pressure. For example P o 2 represents partial pressure of oxygen.
A container may be filled with a number of different gases. The overall pressure of a gas mixture is the sum of the partial pressures of the constituent. Daltons Law of Partial Pressures states that the total pressure of a gas mixture is the sum of the partial pressures of the component gases.
In 1801 English chemist John Dalton made observations about steam and air that is published in 1802 and eventually because Daltons law of partial pressure. The partial pressure of a gas is proportional to its mole fraction χ. At the same time enough oxygen is pumped to make its partial pressure equal to 0432 atm.
The partial pressures of the individual gases in the container. Ptotal P1 P2 P3. The partial pressure is the pressure each gas would exert if it alone occupied the volume of the mixture.
P pa pw 1 where. Anne Marie Helmenstine PhD. However that does not mean Daltons laws validity is dependent on whether pressure is intensive in your system.
The pressure exerted by each gas is called. Updated on September 09 2019. According to the law of Dalton we can conclude that a mixture filled with multiple gases exert partial pressures on each other.
If you have a 50-50 gas mixture in a balloon its. Both Amagats and Daltons laws predict the properties of gas mixtures. What is Daltons law of partial pressures An explanation.
The total pressure equals the sum of partial pressures that each gas exerts. As Dalton understood partial pressures the phenomenon was named after him. People found this article helpful.
P total P 1 P 2 P 3. P total P 1 P 2 P 3. From Daltons law Associated Articles.

15 12 6 Dalton S Law Of Partial Pressure In Mixtures Of Gases Each Component Gas Behaves Independently Of The Other S In 2022 Ideal Gas Law Molecular Physics Formulas

Dalton S Law Of Partial Pressure Dalton S Law 11th Chemistry Chemistry

Daltons Law Of Partial Pressures Easy Science Dalton S Law Easy Science Organic Chemistry Study

Comments
Post a Comment